Unlocking MS: How an EBV Protein Releases the Immune Brakes
For decades, the link between Epstein-Barr virus (EBV) and multiple sclerosis (MS) has been one of the strongest, yet most perplexing, epidemiological clues in neurology. Nearly everyone is infected with this common herpesvirus, but only a small fraction develops MS. The central question has remained: if EBV is a trigger, why does the disease manifest in so few?
Groundbreaking research led by Dr. Nicholas Sanderson and Professor Tobias Derfuss at the University of Basel, published in Cell, now provides a compelling mechanistic answer. Their work moves beyond correlation to describe a precise sequence of rare biological events-a "perfect storm"-that can ignite MS-like damage in the brain.
The Crux of the Paradox: Ubiquitous Infection vs. Rare Disease
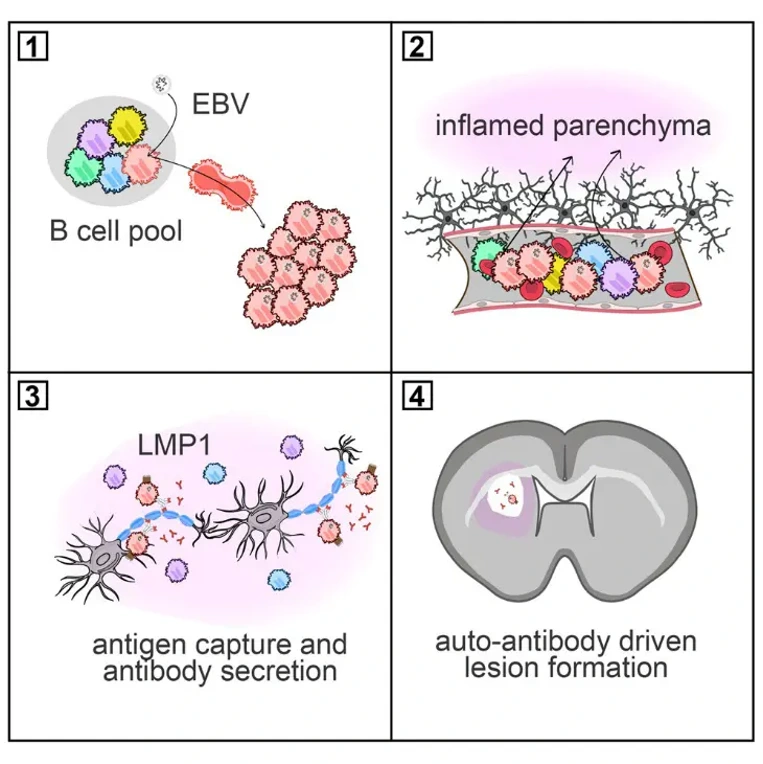
The near-universality of EBV infection has long been a major argument against its direct pathogenic role. The new study reconciles this paradox by shifting the focus from the virus itself to the exceptional and precarious context in which it acts. The research team, bridging clinical neurology and fundamental immunology, zoomed in on B cells. These immune cells are not only the primary reservoir for EBV but are also known to be critical players in MS, as evidenced by the striking efficacy of B cell-depleting therapies.
The Breaking of Tolerance: A Viral Bypass
The key discovery lies in how EBV subverts a fundamental safeguard: B cell tolerance. Our bodies naturally harbor B cells with potential self-reactivity. Under strict regulation, these cells are usually eliminated or rendered harmless upon encountering self-tissue. The Basel team discovered that an EBV protein acts as a molecular decoy, mimicking a crucial survival signal (a "checkpoint") that B cells normally receive from other immune cells.
This viral interference provides a lifeline to self-reactive B cells that should have been silenced. As senior author Tobias Derfuss explains, "We have identified a sequence of events, including EBV infection, that must occur in a well-defined order to spark a local inflammation in the brain." This represents a direct, virus-mediated breach of immune tolerance.
The Perfect Storm in a Privileged Site
A breached tolerance alone is not enough. The brain, an immunologically privileged site, provides the critical second act. During minor inflammation or infection, such virus-manipulated B cells can transiently enter brain tissue. Here, if they encounter their specific self-target-such as components of the myelin sheath-the stage is set for disaster.
In experimental models, this precise scenario led to focal damage to the myelin insulation, strikingly reminiscent of early MS lesions. The process is notably local and specific, not a widespread immune attack. "It's a spark", says Derfuss, highlighting that this mechanism could initiate the chronic inflammation characteristic of MS long before clinical symptoms appear.
Implications for the Future
This research fundamentally shifts the therapeutic gaze earlier in the disease timeline, to a phase when risk might still be malleable. It underscores that MS risk is shaped by a confluence of factors: immune history (EBV infection), genetic predisposition (affecting B cell regulation), and timing of immune-brain interactions.
The most profound implication is for prevention. By defining the initial spark, the work strengthens the rationale for strategies aimed at intercepting the disease process before it becomes entrenched. A vaccine against EBV emerges as a plausible primary prevention tool, not to prevent infection per se, but to prevent the severe or dysregulated viral latency that sets the stage for B cell manipulation. Furthermore, it opens avenues for monitoring high-risk individuals for early signs of this pathogenic B cell activity.
Creative Bioarray is the leading preclinical CRO specializing in animal models services. We have a wide range of transgenic and inducible mouse and rat models readily available for your studies. Our team of animal model scientists will work with you to find the right model.
Learn more about our fully characterized and validated animal models.
Reference
- Kim, Hyein, et al. "Myelin antigen capture in the CNS by B cells expressing EBV latent membrane protein 1 leads to demyelinating lesion formation." Cell (2026).

Your email address will not be published. Required fields are marked *