Beyond Animal Models: A New Era in Biomedical Research
For decades, the path from laboratory discovery to a clinically approved therapy ran through a seemingly indispensable checkpoint: the animal model. It has been the cornerstone of biomedical research, a "gold standard" for assessing safety and efficacy. However, a stark reality persists-despite this reliance, approximately 86% of candidate drugs still fail in human clinical trials, often due to unpredicted toxicity or a lack of efficacy that animal models did not forecast.
This high attrition rate, coupled with profound ethical considerations and rising costs, is driving a pivotal shift in scientific philosophy and practice. As argued in a recent commentary in Nature, we are witnessing the systematic emergence of a new paradigm built not on cross-species extrapolation, but on human-specific biology. This revolution is powered by a suite of technologies collectively known as Novel Alternative Methods/New Approach Methodologies (NAMs).
NAMs represent a fundamental reimagining of the experimental process. They move away from observing complex, often poorly understood reactions in an entire living animal, and instead focus on building highly controlled, human-relevant systems-from engineered tissues to sophisticated computer simulations. The goal is not merely to reduce animal use, but to build more predictive, efficient, and ethically sound models of human biology and disease.
Deconstructing the Black Box: The Toolkit of NAMs
NAMs are not a single tool, but an integrated toolkit designed to answer specific biological questions with human precision. Their power lies in moving beyond the "black box" of a whole organism to dissect mechanisms at the tissue, cellular, and molecular levels.
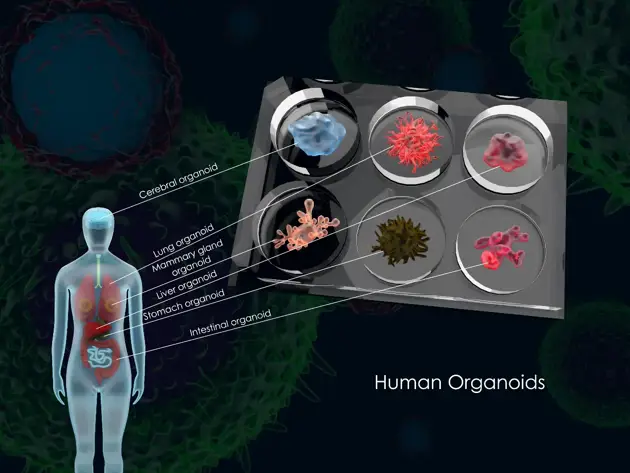
1. Organoids and Advanced 3D Models
Perhaps the most visually compelling NAMs are organoids-miniature, self-organizing 3D structures derived from human stem cells. Unlike traditional cell lines grown flat on a dish, organoids can mimic the complex architecture and cellular diversity of organs like the brain, heart, liver, or a patient's specific tumor. For instance, tumor organoids retain the genetic and microenvironmental hallmarks of the original cancer, providing a powerful platform for personalized drug sensitivity testing. Research shows these "patients-in-a-dish" can predict clinical responses more accurately than standard animal xenograft models in certain contexts.
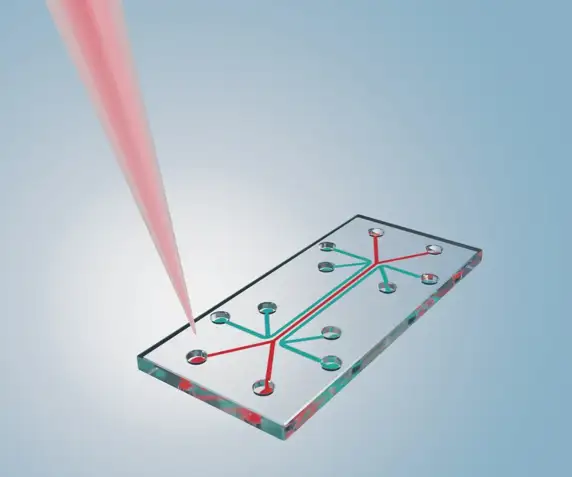
2. Organs-on-Chips and Microphysiological Systems
To add dynamic function to 3D structure, scientists are developing microfluidic "organs-on-chips." These devices, often no larger than a USB stick, contain living human cells arranged to simulate the minimal functional unit of an organ. Channels mimic blood flow, applying physiological shear stress, while engineered membranes allow for the study of barrier functions. A blood-brain-barrier-on-a-chip, for example, can precisely measure a drug's ability to cross into the brain, a critical factor in neurology and neuro-oncology. These systems are now being linked to create "human-body-on-a-chip" platforms to study systemic drug effects.
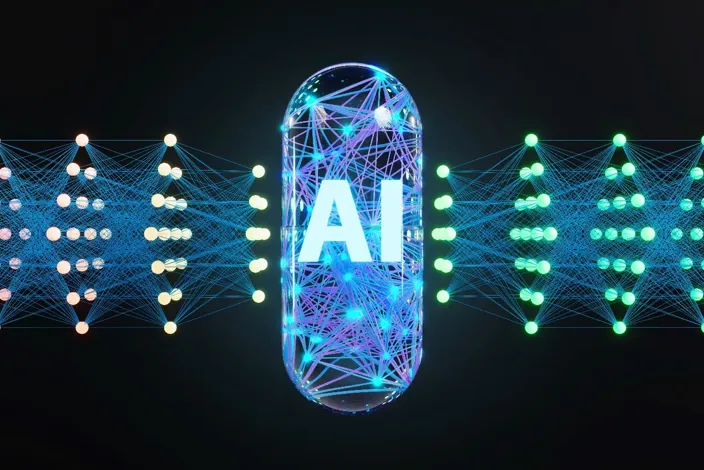
3. In Silico and AI-Driven Models
The most scalable NAMs exist entirely in the digital realm. Computational toxicology uses quantitative structure-activity relationship (QSAR) models to predict a molecule's potential hazard based on its chemical properties. More recently, advanced artificial intelligence models, like the FDA's exploratory AnimalGAN, aim to generate synthetic biological data that can inform safety assessments. These in silico approaches allow for the rapid, cost-effective screening of thousands of compounds, prioritizing only the most promising for further testing in biological systems.
The ultimate vision emerging from this toolkit is the concept of the "clinical trial in a dish". By testing drugs on organoids or cells derived from hundreds or thousands of individuals with diverse genetic backgrounds, researchers can simulate population-wide responses in vitro. This approach holds extraordinary promise for understanding variable drug responses, studying rare genetic diseases, and advancing the goals of precision medicine.
How Policy is Catalyzing the Transition
The scientific promise of NAMs is now being matched by concrete action from key institutions that govern research. A synchronized push from regulatory, funding, and publishing bodies is creating an essential runway for these methods to take off.
1. Regulatory Acceptance
The FDA has been a critical driver. Following the passage of the FDA Modernization Act 3.0, the agency has signaled a historic shift, no longer mandating animal testing for all new drug development pathways. In specific areas, such as for certain monoclonal antibody therapies, sponsors can now submit data from validated human-relevant NAMs. This policy evolution officially recognizes that human biology-based data can provide evidence of safety suitable for regulatory decision-making.
2. Funding and Infrastructure
Substantial investment is following this regulatory lead. The U.S. National Institutes of Health (NIH) has committed $87 million to establish Standardized Organoid Modeling Centers. This national resource is explicitly tasked with developing, benchmarking, and disseminating robust organoid models to reduce reliance on animal studies. Furthermore, the NIH's ORIVA office is working to address systemic bias in grant review by training study sections to properly evaluate proposals centered on NAMs.
3. Publishing Standards
The scientific literature itself is undergoing a transformation. A coalition of major publishers, including Nature, Cell, and The BMJ, have adopted the MISS-NAM reporting standards. A key change is in editorial mindset: the default question for authors is no longer "Why haven't you used an animal model?" but rather "Is the use of an animal model scientifically justified?" This shift is actively protecting early-career researchers from what was once a common and often unnecessary request to "add animal data", thereby accelerating the publication and dissemination of high-quality NAMs research.
From Novelty to New Standard: The Road Ahead
The momentum behind NAMs is undeniable, yet the journey from promising alternative to established standard continues. Key challenges remain, including the need for further technical validation, standardization of protocols, and broader education within the scientific community. The full complexity of organism-level systems biology, particularly neurobiology and immunology, presents a high bar for in vitro models to reach.
However, the direction of travel is clear. The convergence of scientific innovation, regulatory adaptation, and institutional support is creating a powerful feedback loop. NAMs are transitioning from exploratory tools used in a few specialized labs to cornerstone methodologies supported by national centers and accepted by regulators.
This is more than a technical upgrade; it is a foundational shift in how we understand human biology and develop medicines. By building our research on a bedrock of human-relevant systems, we are not just replacing animals-we are building a more predictive, efficient, and ethically aligned framework for 21st-century biomedicine. The future of discovery lies not in translating from mice to humans, but in modeling humanity itself, from the molecule up.
Creative Bioarray offers a comprehensive range of products and services for 3D spheroid and organoid culture, enabling to create more physiologically relevant in vitro models. Our specialized culture media and scaffolds support the growth and development of multicellular structures, while our organoid culture kits allow the establishment and maintenance of organoid models derived from various tissues and cell types.
Moreover, our drug testing platform integrates 3D spheroid and organoid culture models with high-throughput screening technologies to assess the efficacy and safety of pharmaceutical compounds. By mimicking in vivo environments, our innovative solutions provide more predictive results for drug development and toxicity testing.
3D Spheroid & Organoid Culture Reagents
3D Spheroid Platform for Drug Development
Organoid Platform for Drug Development
Organ-on-a-Chip Platform for Drug Development
Genetic Engineering in Organoids
Reference
- Herron, Todd J., et al. "Alternatives to animal testing are the future-it's time that journals, funders and scientists embrace them." Nature 646.8086 (2025): 799-801.

Your email address will not be published. Required fields are marked *