How Synaptic Priming Could Transform Antidepressant Therapy?
Ketamine and dexketamine rapidly relieve depression, with effects occurring within hours of a single dose and their effects often persisting for days to weeks after drug elimination. They are not only effective for treatment-resistant depression but also have potential applications in a variety of psychiatric conditions, including bipolar disorder, anxiety, and PTSD. However, the mechanisms underlying their prolonged effects, which far exceed the drug's half-life, remain largely unknown.
Recently, Todd D. Gould of the University of Maryland published a related research paper in Neuron. This article proposes a "synaptic priming" hypothesis: ketamine does not directly repair the brain but rather, like a "flip switch", activates synaptic plasticity mechanisms, shifting the brain into a responsive state. This state persists after drug withdrawal, making the neural networks more sensitive to subsequent stimulation (such as the next dose or psychotherapy), thus explaining its long-lasting and cumulative effects. This framework also reveals that dosing intervals and environmental factors (e.g., stress or psychological support) can influence the "priming" effect. Appropriately designed intermittent dosing schedules or incorporating positive contextual interventions can enhance efficacy, particularly in poor responders. Furthermore, this mechanism also applies to other plasticity-enhancing drugs, such as psilocybin.
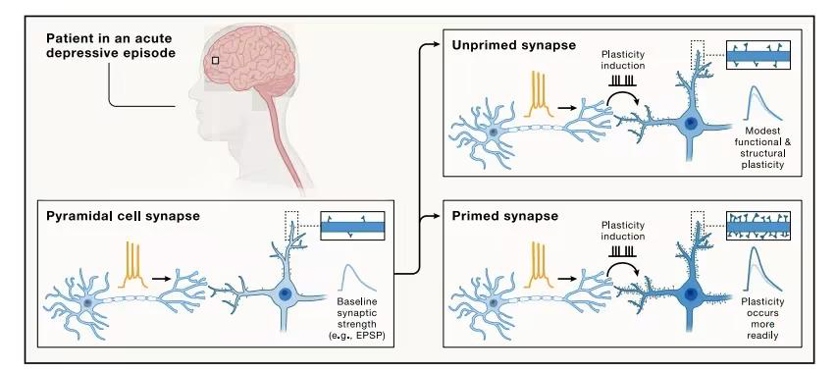
The timing, intensity, and frequency of stimulation significantly influence the responses of brain synapses. Research has shown that specific patterns of stimulation (such as electrical activity, stress, or drugs) can "prime" synapses, causing them to respond more strongly or less strongly to subsequent responses to the same or different stimuli, with effects that can last for hours to days. NMDA receptors are key regulators of this process, altering synaptic plasticity both homosynaptically and heterosynaptically. This "synaptic priming" mechanism is not only involved in learning and memory but also influences neural network stability. Therefore, when treating disorders associated with abnormal plasticity, such as depression, it is crucial to prioritize the temporal and spatial patterning of therapeutic stimulation (e.g., drugs, electrical stimulation) to optimize efficacy.
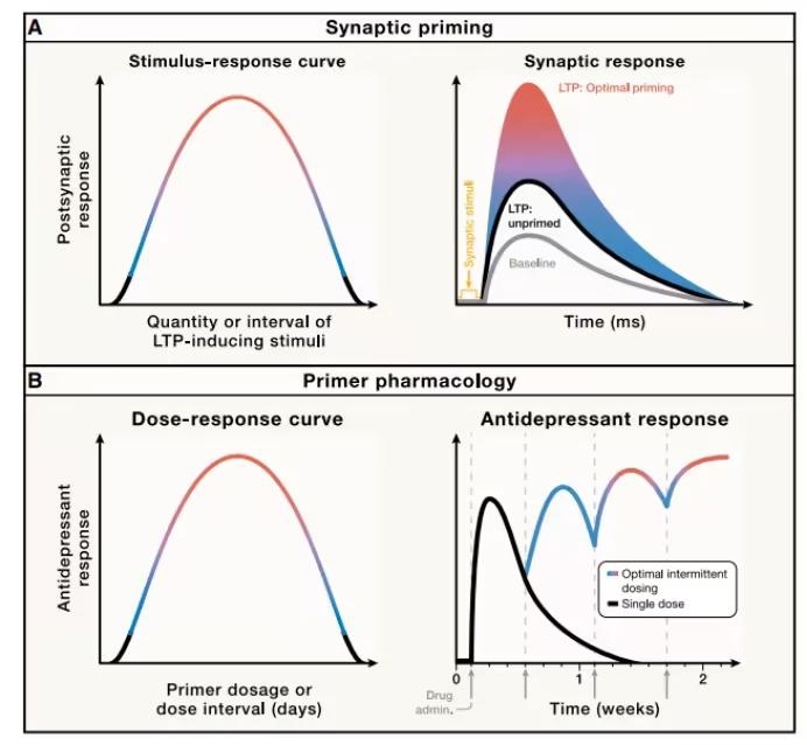
Synaptic potentiation exhibits an inverted U-shaped dose-response relationship: too little stimulation is ineffective, while too much can reverse the potentiation effect. Furthermore, the interval between stimulations is crucial; appropriately prolonging the interval significantly enhances the second potentiation effect, demonstrating that synapses require time to "prime" and prepare. This principle suggests that plasticity-enhancing antidepressants such as ketamine should follow a similar pattern: overly frequent dosing may weaken efficacy, while appropriately lengthening the dosing interval can maximize their antidepressant effect by leveraging the "synaptic priming" mechanism. Therefore, the key to optimizing intermittent dosing regimens lies in understanding the drug's priming effect and timing window, avoiding excessive intervention that could inhibit plasticity.
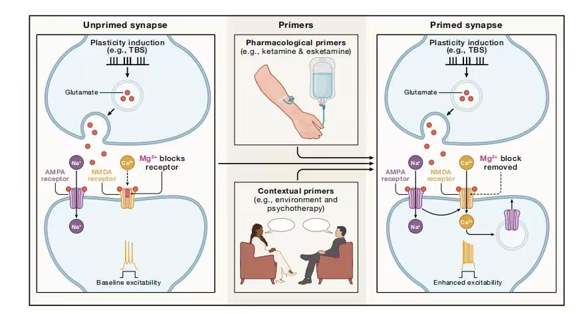
Research has shown that ketamine's antidepressant effects are significantly influenced by the timing and dosage pattern of administration. Its mechanism of action conforms to the classic BCM (Bienenstock-Cooper-Munro) model of plasticity: by "priming" synapses, it persistently alters the brain's ability to respond to subsequent stimulation, thereby reversing the "synaptic rigidification" seen in depression. This framework suggests that ideal treatments should moderately prime plasticity. Overly frequent or excessive dosing could raise the potentiation threshold and weaken efficacy. Therefore, optimizing intermittent dosing intervals is crucial. This principle has successfully guided physical therapies such as repetitive transcranial magnetic stimulation and also offers new insights into the precise use of drugs like ketamine: combining it with personalized brain stimulation can synergistically enhance therapeutic efficacy.
In summary, "synaptic priming" offers new insights into optimizing ketamine treatment strategies and improving antidepressant efficacy, opening up new avenues for future drug development.
We are the leading preclinical neuroscience CRO specializing in animal models of CNS diseases. We have a wide range of transgenic and inducible mouse and rat models readily available for your studies. Our team of animal model scientists will work with you to find the right model.
Learn more about our fully characterized and validated animal models for neurological diseases.
Reference
- Brown, Kyle A., et al. "Synaptic priming: A framework for pharmacotherapy in depression." Neuron (2025).

Your email address will not be published. Required fields are marked *