Triggering Cuprotosis to Fight Leukemia
It is well known that multicellular organisms undergo various predetermined and precisely controlled programmed cell death during development, such as apoptosis, necroptosis, pyroptosis, and ferroptosis.
On March 17, 2022, a team led by Professor Todd Golub at the Broad Institute of Harvard and MIT published a paper in Science. This study revealed for the first time a copper-dependent cell death mechanism, which they named Cuprotosis. This copper-dependent cell death occurs through the direct binding of copper ions to lipoylated components of the tricarboxylic acid cycle (TCA) in mitochondrial respiration, leading to the aggregation of lipoylated proteins and subsequent downregulation of iron-sulfur cluster proteins, resulting in proteotoxic stress and ultimately cell death.
On November 19, 2025, a team led by Lev M. Kats at the University of Melbourne published a study titled "Inhibition of heme biosynthesis triggers cuproptosis in acute myeloid leukemia" in Cell. This study found that inhibition of heme biosynthesis induces copper accumulation in cells of acute myeloid leukemia (AML) and activates cuproptosis.
This research further substantiates cuproptosis as a bona fide cell death pathway and identifies heme biosynthesis enzyme (HBE) as a promising drug target in AML. Inhibiting HBE can kill leukemia cells by activating cuproptosis. This finding provides a new direction for metabolic targeted therapy of AML.
Acute myeloid leukemia is a highly aggressive and deadly cancer with an overall 5-year survival rate of less than 30%. AML is caused by a combination of cancer-driving mutations that accumulate in an individual's hematopoietic stem cells, pluripotent progenitor cells, and/or early myeloid progenitor cells.
Metabolic dysregulation is a hallmark of AML pathogenesis. Metabolic reprogramming not only powers the rapid proliferation of AML cells but also alters their cell fate by regulating the activity of epigenetic and transcriptional circuits. Importantly, this provides actionable therapeutic evidence, and metabolic pathways have proven to be fertile ground for AML drug development. Indeed, metabolic-related therapies targeting nucleotide metabolism (cytarabine), mitochondrial respiration (veneclade and azacitidine), or the production of the oncometabolite 2-hydroxyglutarate (mutant isocitrate dehydrogenase inhibitors) have been used in most AML patients.
Although these therapies can elicit strong responses, even achieve complete remission in some cases, drug resistance and relapse remain common, highlighting the urgent need for novel treatments.
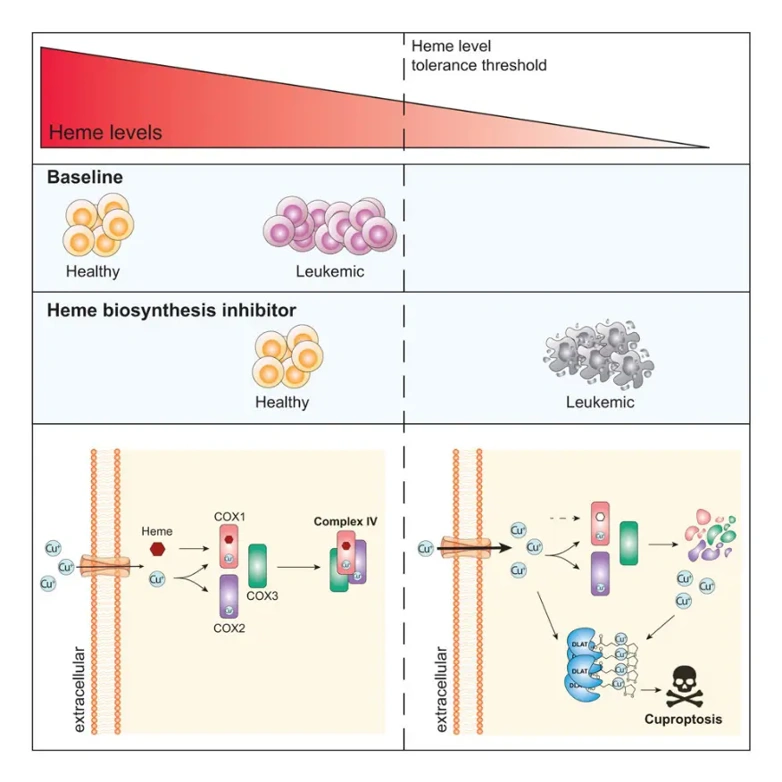
Heme is an essential metabolite with a wide range of biological activities. Cells can acquire heme either by recycling it from the extracellular environment or through de novo synthesis from succinyl-CoA and glycine via a series of eight enzymatic reactions catalyzed by heme biosynthesis enzymes (HBEs) located in mitochondria and cytoplasm. Besides its role as an oxygen carrier in erythrocytes, heme is necessary for and regulates numerous molecular processes in non-erythrocytes, ranging from mitochondrial energy production to iron homeostasis, antioxidant defense, kinase signaling, and transcription. Heme levels have been shown to regulate cell fate determination, including cell differentiation and apoptotic cell death.
However, despite the potential for alterations in heme biosynthesis to affect key characteristics of cancer cells, HBEs remain relatively underexplored as therapeutic targets in cancer.
Copper, an essential trace metal element for life, is also highly toxic; therefore, intracellular copper levels are actively maintained at extremely low concentrations to prevent harmful effects. The copper ionophore Elesclomol can transport copper ions into cells, selectively targeting and killing cancer cells. However, it has not been successful in clinical trials, partly due to insufficient understanding of the toxic mechanisms of copper.
In 2023, Professor Todd Golub's team took a significant step forward by providing a rationale for harnessing copper's therapeutic potential. They described a form of copper-induced cell death-cuprotosis-which is distinct from known pathways like apoptosis, necroptosis, and ferroptosis. Cuprotosis involves two key factors: 1) excessive accumulation of copper ions, usually induced by ionophores; and 2) mitochondrial protein complexes, such as pyruvate dehydrogenase, that contain a lipoic acid-derived post-translational modification (lipoylation). Copper directly binds to lipoylated proteins, inducing oligomerization and triggering lethal protein toxicity stress.
Notably, copper ionophores have shown high activity in vitro against certain AML subtypes with poor prognosis.
Lev M. Kats' team has previously found that HBE expression and heme levels are reduced in AML, combined with analysis of the Cancer Dependency Map (DepMap) indicated that HBE is a highly selectively dependent factor in AML. Therefore, the team explored the feasibility of targeting HBE as an anti-leukemia strategy.
Specifically, through a comprehensive analysis of mouse models, human cell lines, and primary patient samples, this study found that de novo heme biosynthesis is a selective survival dependency in AML. The underlying mechanism for this dependency lies in the fact that AML cells (especially leukemia stem cells, LSCs) tend to downregulate the expression of HBE, and this low HBE state actually promotes their self-renewal capacity.
Inhibition of HBEs leads to: 1) collapse of mitochondrial complex IV, disrupting oxidative phosphorylation; and 2) dysregulation of the copper chaperone protein system, causing an imbalance in cellular copper homeostasis and thereby inducing cuprotosis.
Furthermore, this study identified pathways with synthetic lethal relattionships to heme biosynthesis (such as glycolysis), suggesting potential targets for combination therapy strategies.
Reference:
- Lewis, Alexander C., et al. "Inhibition of heme biosynthesis triggers cuproptosis in acute myeloid leukaemia." bioRxiv (2024): 2024-08.

Your email address will not be published. Required fields are marked *