Science's Tiny Drug Delivery Robots
When you take a pill, only a minimal amount of the drug ultimately reaches the diseased site, while the majority distributes throughout the body. This systemic administration often leads to off-target effects, which not only limit therapeutic efficacy but also potentially increase toxic side effects. In fact, about one-third of all developed drugs fail to gain market approval due to excessive side effects.
For decades, researchers have been exploring the use of microrobots to precisely deliver therapeutic agents to the diseased site. This targeted drug delivery method aims to enhance local drug concentration at the lesion while minimizing systemic drug exposure, thus reducing potential adverse effects.
On November 13, 2025, researchers from ETH Zurich published a study titled: "Clinically ready magnetic microrobots for targeted therapies" in Science.
The study developed a clinical-grade magnetic microrobot, roughly the size of a grain of sand (less than 2 mm in diameter). This microrobot can navigate within blood vessels under magnetic guidance to deliver drugs precisely to specific sites, thereby avoiding the toxic side effects associated with systemic therapy and providing a promising solution for precise targeted drug delivery.
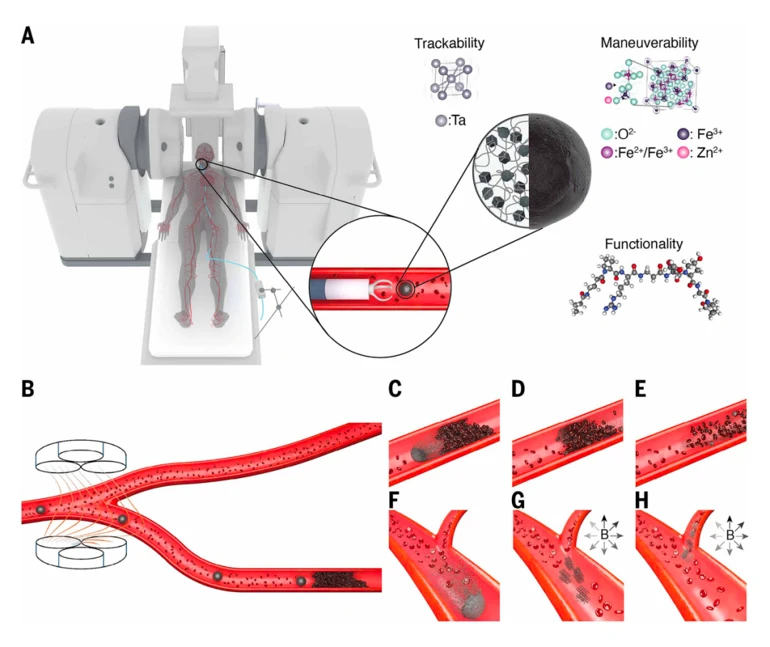
While microrobots have shown great potential in targeted drug delivery over the past two decades, clinical translation has been slow. The issue lies in the often fragmented nature of existing research-key technologies such as motion control, drug loading, and real-time imaging are studied separately, lacking integration.
The platform launched by this team achieves three major breakthroughs:
-
Modular design. The platform seamlessly integrates an electromagnetic navigation system, a customized delivery catheter, and a magnetic microrobot, allowing for flexible adaptation to different clinical scenarios. This design allows hospitals to use it without large-scale modifications to existing facilities.
-
Clinical-grade navigation system. The team employed a dual Navion electromagnetic navigation system to generate a 20×20×20 cm workspace sufficient to cover the human head, with a magnetic field gradient of up to 1 T/m, ensuring stable navigation of the microrobot within blood vessels.
-
Safe and biodegradable microrobot. The robot is made of a gelatin matrix containing iron oxide nanoparticles (for magnetic response), tantalum nanoparticles (visible under X-ray), and therapeutic drugs. All materials are FDA-approved for intravascular application, ensuring safety.
The spherical microrobot has a diameter of approximately 1.69 mm, suitable for navigating the cerebral vasculature of porcine models, which is similar in size to human brain vessels.
The team designed three navigation modes to adapt to different blood flow environments:
(1) Rotational Rolling: Rolling along the blood vessel wall, suitable for low-flow areas;
(2) Countercurrent Navigation: Against blood flow, capable of reversing flow up to 21.2 cm/s;
(3) Co-current Navigation: Utilizing blood flow for transport, with magnetic gradients guiding direction, achieving a 95% success rate at vascular bifurcations.
Upon reaching the target site, a 510 kHz high-frequency magnetic field excites the iron oxide particles to generate heat, causing the gelatin matrix to dissolve and release the drug within 40 seconds. This approach achieves both timed and targeted drug delivery and provides a safety mechanism-the drug release is terminated if the microrobot deviates from the target.
Experiments in a biomimetic vascular model were promising: at a flow rate of 37 cm/s in the internal carotid artery (close to the actual blood flow velocity in adults), the microrobot was precisely navigated to different branches of the middle cerebral artery. Targeted thrombolysis demonstrations further showcased its therapeutic potential: microrobots loaded with recombinant tissue plasminogen activator (rtPA) were guided to the thrombus, and drug release via thermal dissolution initiated blood vessel reopen within 7.5 minutes, with the thrombus largely dissolved after 19 minutes.
Large animal studies further validated its clinical feasibility: In a pig model, the team successfully guided the microrobot to target vessels like the facial artery and lingual artery using the three navigation modes under fluoroscopy. In a sheep model, the microrobot even successfully navigated to the fourth ventricle, demonstrating its potential application in the central nervous system.
Although clinical application is still some time away, this research provides a practical technical pathway for precise targeted drug delivery. The platform's advantages lie in its material safety, modular system, and compatibility with existing clinical workflows. In the future, this technology may be used to treat diseases such as vascular occlusion, localized infections, or tumors. By reducing systemic drug exposure, it holds promise for significantly improving efficacy while minimizing side effects.
Reference:
- Landers, Fabian C., et al. "Clinically ready magnetic microrobots for targeted therapies." Science 390.6774 (2025): 710-715.

Your email address will not be published. Required fields are marked *