In Vivo CAR-T: The Game Changer
Over the past three decades, immunotherapy has undergone significant transformation, driven by advancements in monoclonal antibodies, antibody-drug conjugates, cytokines, immune cell connectors, DNA vaccines, and more recently, RNA vaccines and engineered T-cell therapies.
Among these therapeutic modalities, autologous Chimeric Antigen Receptor (CAR) T-cell therapy has emerged as a standout. This approach involves harvesting a patient's own T cells, genetically modifying them to express a CAR, and then reinfusing them into the patient. Its remarkable efficacy, demonstrating curative potential in the treatment of B-cell malignancies, has garnered significant attention.
Despite initial expectations, the expansion of autologous or allogeneic CAR-T cell therapies to a wider range of indications and patient populations has been slower than anticipated. This is due to numerous persistent obstacles, including complex manufacturing and logistics, limited production capacity, and the requirement for chemotherapy-based lymphodepleting pretreatment, all of which constrain its accessibility and applicability.
In vivo CAR-T cell engineering, which aims to generate CAR-T cells directly within the patient's body, strives to overcome the aforementioned challenges by eliminating the need for ex vivo cell processing and complex logistics, while simultaneously improving clinical efficacy.
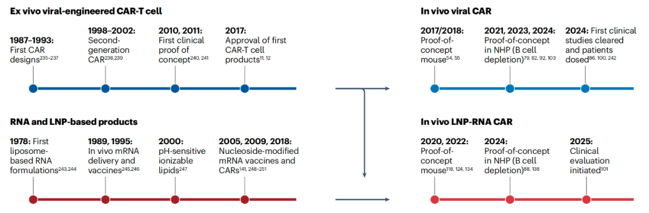
Recent advances in virology, RNA therapeutics, and nanotechnology have fundamentally revolutionized in vivo CAR-T, using targeted delivery systems such as lentiviral vectors and lipid nanoparticles (LNPs) to introduce CAR-encoding genetic material into endogenous T cells.
Currently, two primary in vivo CAR platforms are advancing towards clinical translation: engineered viral (lentivirus, gamma-retrovirus) vectors, which integrate the payload into the host genome; and lipid nanoparticles (LNPs), which enable transient expression of the payload within host cells.
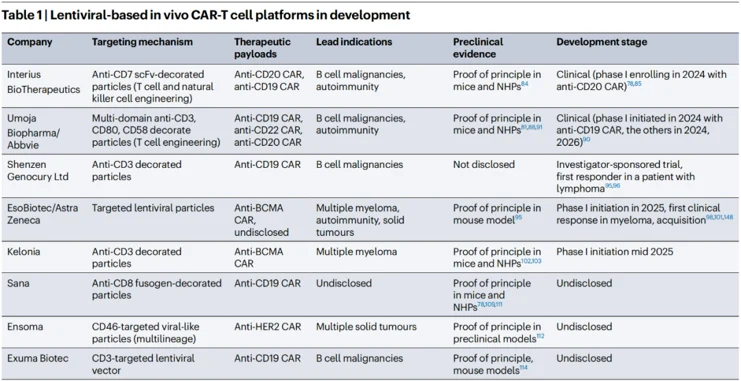
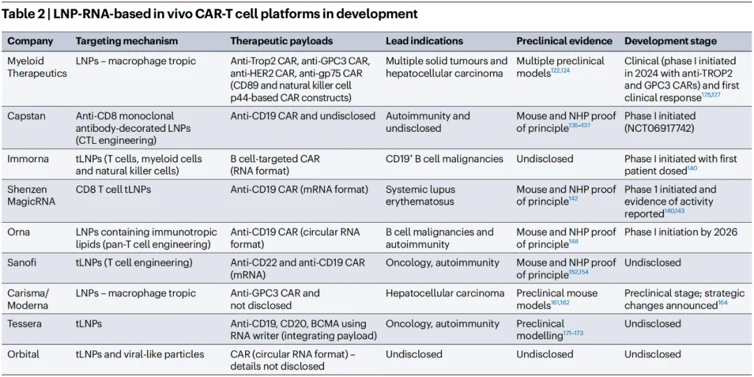
Several candidates, including both engineered viruses and LNP-RNA formulations, are now progressing clinically. Following initial demonstrations of safety, tolerability, and clinically meaningful biological activity by the first candidates, they are expected to rapidly advance to proof-of-concept and subsequent registration trials. This will be accompanied by an expansion of clinical activity across multiple indications in oncology and autoimmunity. Given the novelty of these platforms and their yet-to-be-defined therapeutic index, most candidates will likely be initially tested in oncology indications, followed by applications in autoimmune diseases or regenerative medicine. As the technology continues to evolve, a diversification of product concepts, payload architectures, and targeted delivery vectors are anticipated, paving the way for increasingly innovative and disruptive in vivo therapies for diseases difficult to treat with conventional techniques.
Reference:
- Bot, A., Scharenberg, A., Friedman, K. et al. In vivo chimeric antigen receptor (CAR)-T cell therapy. Nat Rev Drug Discov (2025).

Your email address will not be published. Required fields are marked *