How Microglia and Astrocytes Shape Synapse Remodeling
In a new study, a research team led by Dr. Dorothy P. Schafer and Dr. Travis E. Faust of the University of Massachusetts Chan School of Medicine explains how two distinct cell types in the brain—astrocytes and microglia—communicate in response to changes in sensory input to reshape connections between neurons, known as synapses.
The findings, published in the journal Cell, represent an emerging area of interest for neurobiologists seeking to understand how different cells in the brain interact to rewire the brain.
This novel mechanism has the potential to become a target for translational scientists who hope to one day prevent synaptic damage that occurs during neurodegenerative diseases and age-related cognitive decline. It may also provide new insights into neurodevelopmental and psychiatric disorders, such as autism and schizophrenia, in which the brain's circuit refinement may be impaired during development.
"The brain is made up of billions of cells, and these cells must somehow coordinate with each other for proper brain connectivity," said Dr. Schafer. "Understanding how all these cells coordinate is key. Here, we provide new insights into how non-neuronal cells called glia communicate with each other to rewire brain circuits during development. We believe this is relevant to many neurodegenerative and neurodevelopmental diseases, in which brain circuits are inappropriately remodeled."
As the brain develops, neural circuits are finely tuned and pruned in response to sensory stimuli received from the environment. Dr. Faust, a co-author of the study, explained that if too many synapses are allowed to form, neural networks cannot function properly.
Autism is a neurodevelopmental disorder believed to be caused by insufficient synaptic pruning. Schizophrenia has been found to have too few synapses in some parts of the brain and too many in others.
Conversely, many neurodegenerative diseases, such as Alzheimer's disease and ALS, are characterized by the degeneration and destruction of synapses, and unchecked synaptic remodeling is believed to contribute to cognitive and functional decline. Protecting these synapses from dysregulated synaptic remodeling may prove beneficial for patients with these diseases.
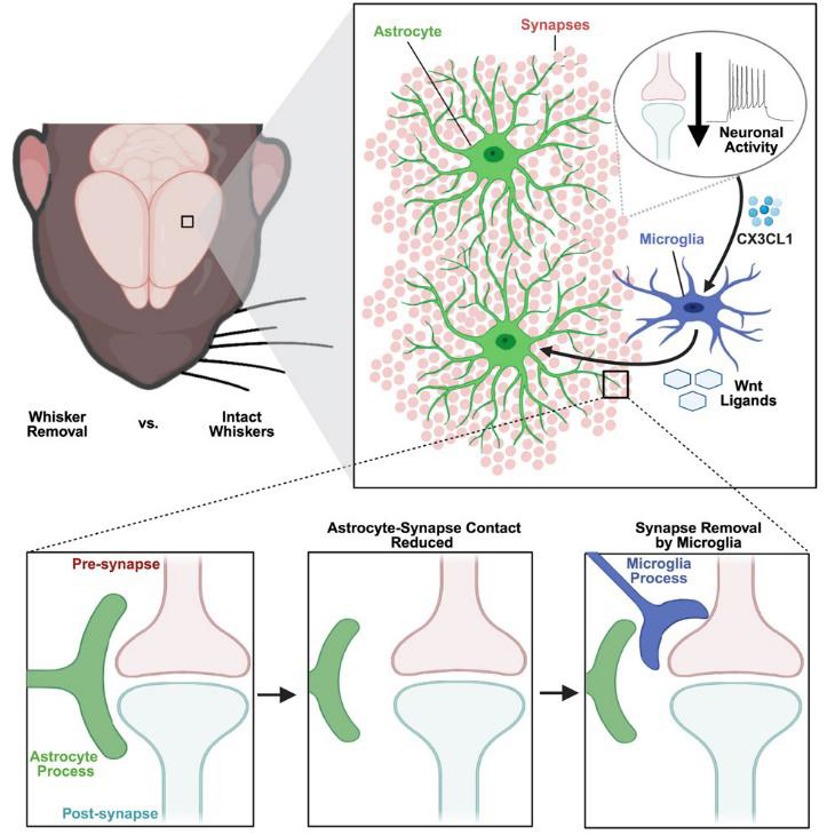
"This study demonstrates how two different subtypes of glial cells—astrocytes and microglia—work together to remove, or 'prune,' synapses in the cerebral cortex following altered sensory experience," said Schafer.
This research builds on a previous study from the Schafer lab, published in 2019 in the Journal of Neuroscience, in which they altered the sensory experience of developing mice. "Even during early development, subtle changes in an animal's sensory experience can have profound effects on brain connectivity," said Schafer.
Schafer and Faust now reveal how this rewiring is achieved. "You can imagine astrocytes as a dense bush with millions of tiny branches that contact individual synapses and fill all the gaps between neurons in the brain," said Schafer.
In contrast, microglia are resident immune cells that monitor the brain and typically engulf synapses. "We discovered that microglia secrete molecules called Wnts that signal astrocytes. This signaling causes astrocytes to move their tiny branches away from synapses, allowing microglia to quickly enter and engulf those synapses," Faust said.
The key messenger molecules, Wnts, are a family of cell-secreted proteins that are crucial for many biological processes, including embryonic development, tissue homeostasis, stem cell regulation, and cell differentiation. In this case, Wnt proteins act as a signal, telling astrocytes to move away and expose synapses. This frees microglia to enter and engulf those exposed, inactive synapses.
The implications of these findings are far-reaching. "The brain undergoes a lot of remodeling during development as our sensory experiences change as we experience the world around us," said Schafer. "It's conceivable that similar things happen in other contexts, such as during learning and memory, or during sleep. We are also investigating whether this might be involved in disorders such as autism and schizophrenia, where these remodeling processes can go awry or be abnormally upregulated at inappropriate times and places, leading to the loss of synaptic connections and cognitive decline seen in neurodegenerative diseases like Alzheimer's. Understanding how these processes are involved in disease is an important next step for us."
Accelerate your programs by leveraging Creative Bioarray's extensive inventory and prospective network of cell products.
| Cat. No. | Product Name |
|---|---|
| CSC-7832W | Human Astrocytes-midbrain (HA-mb) |
| CSC-7834W | Human Astrocytes-cerebellar (HA-c) |
| CSC-7835W | Human Astrocytes-brain stem (HA-bs) |
| CSC-7833W | Human Astrocytes-hippocampal (HA-h) |
| CSC-C1527 | Human Microglia |
| CSC-C1800 | Rat Microglia |
| CSC-C9343W | Mouse Microglia |
| CSC-C5456W | RFP Expressing Human Astrocytes (RFP-HAs) |
| CSC-C5479W | GFP Expressing Human Astrocytes (GFP-HAs) |
| CSC-00836L | Human iPSC-derived Astrocytes |
| CSC-00835L | Human iPSC-derived microglia |
| CSC-C12025Z | Immortalized Human Astrocytes-SV40T |
| CSC-I2284Z | Immortalized Human Astrocytes-SV40T-GFP |
| CSC-I9064L | Immortalized Human Astrocytes, fetal-hTERT |
| CSC-I9004L | Immortalized Human Microglia-SV40 |
| CSC-C12028Z | Immortalized Human Microglia-GFP |
| CSC-C12029Z | Immortalized Human Microglia-RFP |
| CSC-I2227Z | Immortalized Mouse Microglia (BV2) |
| CSC-I9208L | Immortalized Mouse Microglia (SIM-A9) |
Reference:
- Faust, Travis E., et al. Microglia-astrocyte crosstalk regulates synapse remodeling via Wnt signaling. Cell 188.19 (2025): 5212-5230.

Your email address will not be published. Required fields are marked *