How 'Star Molecule' CCN1 Orchestrates Astrocyte Functions Across Regions
On December 17, 2025, the team of Joshua E. Burda at Cedars-Sinai Medical Center in Los Angeles, USA, published a research paper in Nature titled "Lesion-remote astrocytes govern microglia-mediated white matter repair". This study used various transcriptomic analysis methods to investigate lesion-remote astrocytes (LRAs) in the spared spinal cord regions of mice after traumatic spinal cord injury. The study found that LRAs acquired a molecularly distinct, neuroanatomically restricted reactive state after spinal cord injury.
Transcriptionally distinct reactive LRAs were found in degenerating white matter, where they guided the specification and function of local microglia, clearing lipid-rich myelin debris and promoting tissue repair. The adaptation of LRA function was facilitated by the secreted matricellular protein CCN1. Loss of astrocyte-derived CCN1 led to excessive and aberrant activation of local microglia, characterized by abnormal molecular specification, impaired debris processing reflected in intracellular accumulation of myelin and axonal debris, and dysregulated lipid metabolism with significantly attenuated lipid droplet accumulation.
Mechanistically, the study found that CCN1 binding to microglial SDC4 increased lipid storage, linking this signaling axis to an important repair-associated lipid buffering response in debris-clearing microglia. Therefore, microglial defects resulting from astrocyte CCN1 depletion ultimately led to delayed white matter debris clearance and impaired neurological recovery after spinal cord injury. White matter astrocytes expressing CCN1 are induced by local myelin damage and are present in various demyelinating diseases in mice and humans, suggesting a fundamental and evolutionarily conserved role in white matter repair.
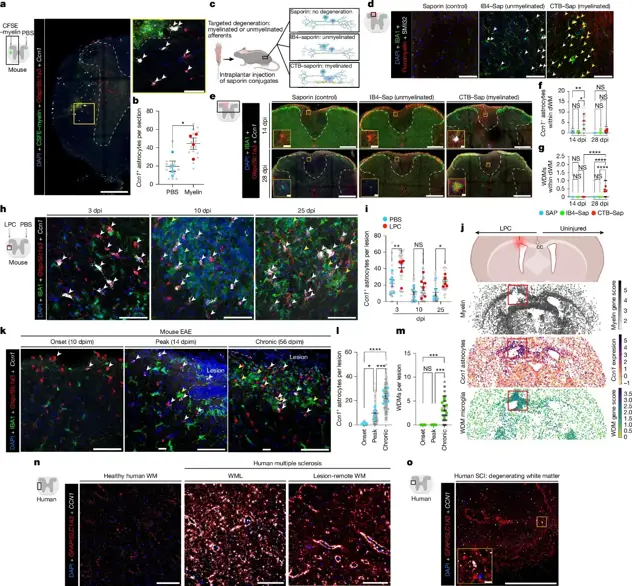
In summary, these findings indicate that environment-specific cues shape regionally distinct LRA reactive states with functional adaptations that coordinate multicellular processes underlying neural repair and influence disease outcomes.
On December 17, 2025, the team of Nicola J. Allen at the Salk Institute for Biological Studies published a research paper in Nature titled "Astrocyte CCN1 stabilizes neural circuits in the adult brain." This study takes a comprehensive approach to address these issues and establishes astrocytes as key coordinators of circuit stability.
Astrocytes, located in the central nervous system (CNS), play a crucial role in maintaining healthy nervous system function, including regulating synaptic development, buffering neurotransmitters and ions, and providing metabolic substrates. In response to various CNS injuries, astrocytes exhibit context-specific transformations, collectively termed reactivity. The characteristics of regionally and molecularly distinct reactive states are not fully understood. The mechanisms by which different reactive states arise, how they evolve or subside over time, and their impact on local cellular function and CNS disease progression remain unclear.
Adjacent to CNS lesions, border-forming astrocytes (BFAs) undergo transcriptional reprogramming and proliferation, forming a neuroprotective barrier that limits inflammation and supports axonal regeneration. Outside the lesion area, undamaged but dynamic regions of the injured CNS exhibit varying degrees of synaptic circuit remodeling and progressive cellular responses to secondary injury, which have profound implications for neural repair and recovery.
In these regions where cellular structures remain intact but exhibit a response to injury, LRAs are interspersed with neurons and glia, show little proliferation, and exhibit varying degrees of cellular hypertrophy. The molecular and functional characteristics of LRAs remain unclear, as does whether regionally restricted microenvironments impose discrete astrocyte reactive states.
This study utilizes an integrated transcriptional analysis approach to identify multiple spatiotemporally resolved, molecularly distinct states of LRA reactivity in the injured spinal cord. It was found that LRAs exhibit transcriptional characteristics distinct from BFAs and astrocytes associated with non-traumatic spinal cord injury and disease. LRA-mediated heterotypic cell interactions, astrocyte-specific gene deletion, and multiple acute and chronic CNS white matter degeneration mouse models were used to reveal and probe previously unidentified white matter LRA reactive states.
The results revealed that this state (1) is caused by focal myelin damage; (2) regulates the molecular, metabolic, and functional characteristics of debris-clearing microglia; and (3) supports nervous system recovery after injury. The results further suggest that CCN1 secreted by white matter LRAs may bind to SDC4 on microglia to enhance their lipid storage activity. Astrocyte-specific CCN1 depletion weakens white matter debris clearance after spinal cord injury (SCI) and impairs nervous system recovery, linking LRA-mediated CCN1 signaling to important repair-related lipid buffering responses in debris-clearing microglia.
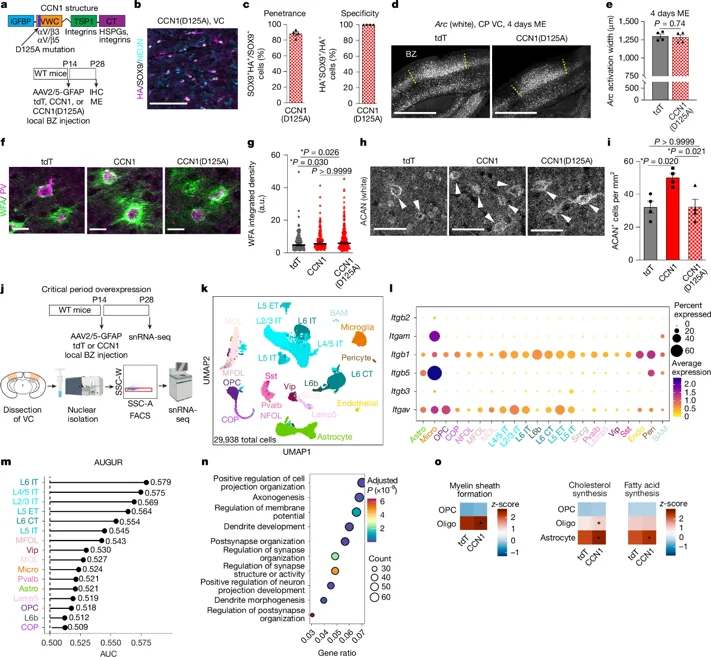
These findings indicate that LRAs are key coordinators of multicellular neurorepair processes that promote functional recovery after central nervous system injury.
Accelerate your programs by leveraging Creative Bioarray's extensive inventory and prospective network of cell products.
| Cat. No. | Product Name |
|---|---|
| CSC-7832W | Human Astrocytes-midbrain (HA-mb) |
| CSC-7834W | Human Astrocytes-cerebellar (HA-c) |
| CSC-7835W | Human Astrocytes-brain stem (HA-bs) |
| CSC-7833W | Human Astrocytes-hippocampal (HA-h) |
| CSC-C1527 | Human Microglia |
| CSC-C1800 | Rat Microglia |
| CSC-C9343W | Mouse Microglia |
| CSC-C5456W | RFP Expressing Human Astrocytes (RFP-HAs) |
| CSC-C5479W | GFP Expressing Human Astrocytes (GFP-HAs) |
| CSC-00836L | Human iPSC-derived Astrocytes |
| CSC-00835L | Human iPSC-derived microglia |
| CSC-C12025Z | Immortalized Human Astrocytes-SV40T |
| CSC-I2284Z | Immortalized Human Astrocytes-SV40T-GFP |
| CSC-I9064L | Immortalized Human Astrocytes, fetal-hTERT |
| CSC-I9004L | Immortalized Human Microglia-SV40 |
| CSC-C12028Z | Immortalized Human Microglia-GFP |
| CSC-C12029Z | Immortalized Human Microglia-RFP |
| CSC-I2227Z | Immortalized Mouse Microglia (BV2) |
| CSC-I9208L | Immortalized Mouse Microglia (SIM-A9) |
References:
- McCallum, Sarah, et al. "Lesion-remote astrocytes govern microglia-mediated white matter repair." Nature (2025): 1-12.
- Sancho, Laura, et al. "Astrocyte CCN1 stabilizes neural circuits in the adult brain." Nature (2025): 1-11.

Your email address will not be published. Required fields are marked *