Ending the Arrhythmia Risk: Science Breakthrough Merges Peptide Scaffold with Nanoelectronics for Stable Heart Repair
Human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs), cultured from a patient's own stem cells, represent a revolutionary approach to repairing hearts damaged by myocardial infarction and heart failure. They can "replenish" necrotic heart muscle, restoring cardiac contractile function, and are considered a core hope in regenerative medicine.
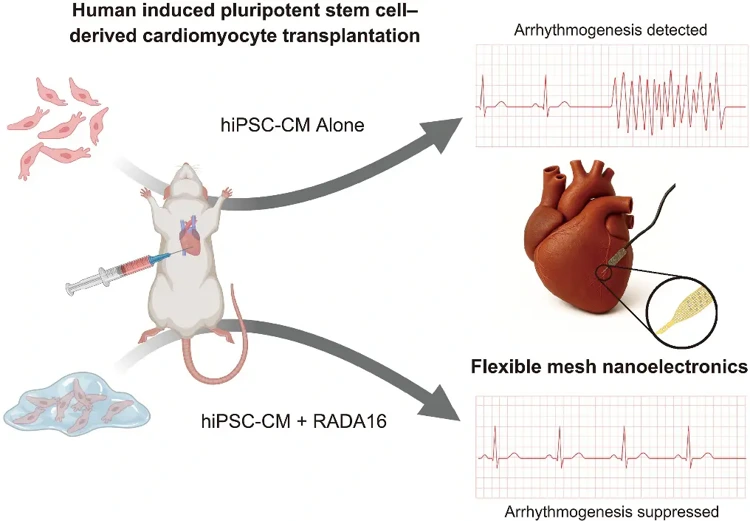
However, a critical bottleneck has long hindered clinical translation: transplanted hiPSC-CMs often cause fatal arrhythmias due to their immaturity and difficulty in synchronizing electrical signals with native cardiomyocytes, making this "life-saving therapy" inherently risky.
Recently, a groundbreaking study published in Science has completely overcome this challenge: a Harvard University team combined the clinically approved self-assembling peptide RADA16 with flexible mesh nanoelectronic technology. This approach allows transplanted cells to mature rapidly and integrate precisely into the native heart, while simultaneously monitoring electrical activity in real time, successfully suppressing arrhythmias and providing a "safety lock" for cardiac regenerative therapy.
For years, stem cell biologists and cardiac researchers have been exploring methods to improve the maturation and integration of hiPSC-CMs. However, the core problem is that once transplanted into the body, the structural development and electrical signal coupling of the cells cannot be directly observed. Only inferred through indirect methods such as surface electrocardiograms, making it difficult to precisely optimize treatment strategies.
The interdisciplinary collaboration between Professor Jia Liu and Professor Richard T. Lee's teams at Harvard University has successfully solved this dilemma: they embedded flexible mesh nanoelectronic devices developed in Liu's laboratory into hiPSC-CMs, creating a "cyborg organoid" platform that enables high-resolution monitoring of transplanted cells for several months.
This flexible nanoelectronic device is a "miniature cardiac monitor"-it is as thin as a cicada's wing and can flexibly deform with the beating heart. Its 32-channel high-density microelectrode array can precisely capture the electrical signals of individual cells and clearly separate the electrical activity characteristics of transplanted cells from the strong signals of native cardiomyocytes, accurately locating the "problem cells" causing asynchronous discharge. "Previously, no technology could directly reveal the dynamics of transplanted cells inside the heart," said Jia Liu. "Our device, combined with computational analysis, enables real-time recording of the electrical activity of transplanted cells within a beating heart, which is an unprecedented breakthrough."
Using this platform, the team tested various optimization strategies, among which the self-assembling peptide RADA16, already clinically approved as a hemostatic agent, showed the most promising results. RADA16 can self-assemble into a nanofiber scaffold in vivo, mimicking the native extracellular matrix of the heart and providing a "growth substrate" for hiPSC-CMs.
The researchers mixed RADA16 with hiPSC-CMs and transplanted them into the left ventricular wall of nude mice. Through immunofluorescence staining and spatial transcriptomics analysis, they found that RADA16 brought three key improvements: First, it promoted angiogenesis, significantly increasing the anastomosis rate of host blood vessels in the transplanted area, with a much higher percentage of CD31-positive blood vessel area compared to the group with hiPSC-CMs alone, providing sufficient oxygen and nutrients to the cells. Second, it accelerated structural maturation, resulting in more regular sarcomere arrangement of hiPSC-CMs, upregulation of adult-type myosin heavy chain (MYH7) expression, and downregulation of fetal-type (MYH6) expression, with sarcomere length and tissue organization at 3 months approaching that of adult myocardium. Third, it enhanced electrical signal coupling, increasing the expression of gap junction protein GJA1, allowing for smoother electrical signal transmission between cells.
More importantly, the results from flexible nanoelectronic monitoring showed that in the control group with hiPSC-CMs alone, significant arrhythmia-like spontaneous discharges were still present after 80 days-these cells beat irregularly at their own rhythm, conflicting with the native myocardial signals; while in the RADA16-treated group, spontaneous discharges were significantly reduced, almost completely disappearing after 135 days, with electrical signals highly synchronized with the native heart rhythm. Power spectral density analysis showed that the electrical signal characteristics of the transplanted area in the RADA16 group were not significantly different from those of the native myocardium, confirming that the transplanted cells were truly integrated into the heart's electrical network.
Further mechanistic studies revealed that RADA16's advantages stem from its dual "bioscaffold + signal regulation" function: it not only provides physical support for hiPSC-CMs but also regulates gene expression by binding to cell surface integrins-inhibiting the pacemaker cell-related HCN4 channel, upregulating the potassium channel KCNJ2, and reducing abnormal automatic pacing; simultaneously promoting the formation of perineuronal nets around inhibitory neurons to stabilize the electrical signal microenvironment. Flexible nanoelectronic technology addresses the "monitoring blind spot", allowing researchers to precisely track the cellular state at every time point, providing direct evidence for optimizing treatment strategies.
"Safety is the biggest obstacle for cardiac cell therapy to enter clinical practice," emphasized Richard T. Lee. "Our research has not only found a solution for arrhythmias but also established an integrated treatment and monitoring system." Notably, RADA16 has already been approved for clinical use, ensuring its safety and eliminating the need for lengthy preclinical toxicity testing, laying the foundation for rapid translation. Furthermore, this platform can be extended to other regenerative therapies-whether liver, nerve, or skin transplantation-wherever cell integration and functional synchronization are involved, precise monitoring can be achieved through embedded nanoelectronics.
The significance of this study extends far beyond technological breakthroughs: it shifts cardiac regeneration from "blindly adding cells" to "precisely repairing the heart", addressing the core problems of slow maturation and poor integration of hiPSC-CMs, while ensuring treatment safety through real-time monitoring. This offers a dual hope of "cell replacement + safety assurance" for patients with myocardial infarction and heart failure. In the future, with technological optimization, "personalized transplantation" is expected to become a reality-extracting the patient's own stem cells, pre-treating them with RADA16, and then monitoring the therapeutic effect in real-time using nanoelectronics to adjust the treatment plan, truly achieving precise and safe cardiac regeneration. For tens of millions of heart disease patients worldwide, this breakthrough makes "heart regeneration" no longer a distant dream, but an imminent clinical reality.
Accelerate your programs by leveraging Creative Bioarray's extensive inventory and prospective network of cell products.
| Cat. No. | Product Name |
|---|---|
| CSC-C2847 | Human Cardiomyocytes |
| CSC-C2847SC | Human iPSC-Derived Cardiomyocytes |
| CSC-I9071L | Immortalized Human Cardiomyocytes-SV40 |
| CSK-IC001 | QualiStem® IPS Cell Cardiomyocyte Differentiation Kit |
Reference:
- Aoyama, Junya, et al. "Flexible nanoelectronics reveal arrhythmogenesis in transplanted human cardiomyocytes." Science 390.6774 (2025): eadw4612.

Your email address will not be published. Required fields are marked *