The Night Shift Risk: Disrupted Circadian Rhythm May Fuel Breast Cancer Metastasis
Working night shifts, frequent transcontinental flights, and staying up late using mobile phones-these behaviors that disrupt sleep patterns are far more than just causing fatigue. Recent research confirms that circadian rhythm disruption (CRD) not only weakens immunity but also directly drives the development and metastasis of aggressive breast cancer.
A groundbreaking study published in Oncogene by Dr. Tapasree Roy Sarkar's team, for the first time, revealed the core mechanism behind this. The team used genetically engineered mouse models (which spontaneously develop triple-negative breast cancer, TNBC) for their experiments: one group of mice maintained a normal 12-hour light/12-hour dark (LD 12:12) cycle, while the other group simulated circadian rhythm disruption caused by shift work or transcontinental flights by advancing the light cycle by 8 hours every two days.
The results were shocking: mice in the normal sleep cycle group showed significant tumors after approximately 22 weeks, while the circadian rhythm disruption group showed signs of cancer at 18 weeks, nearly 20% earlier; even more dangerously, the tumors in the disrupted group were more aggressive, with a significantly increased number of lung metastases, and a significantly lower albumin/globulin ratio (AGR)-a lower AGR indicates a poorer cancer prognosis.
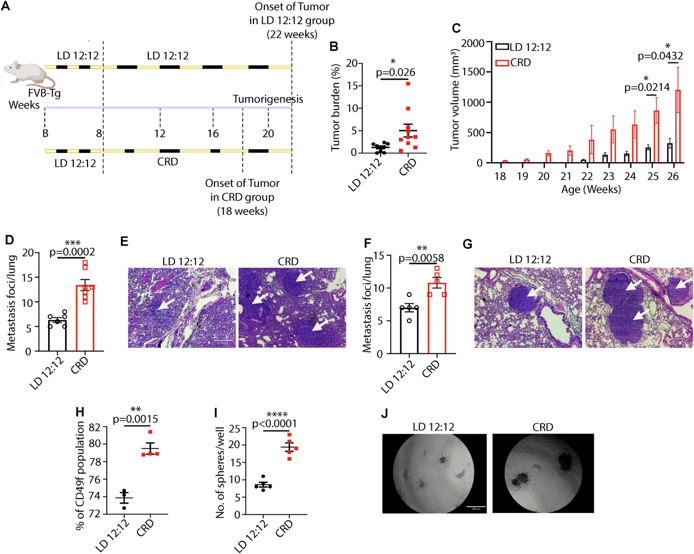
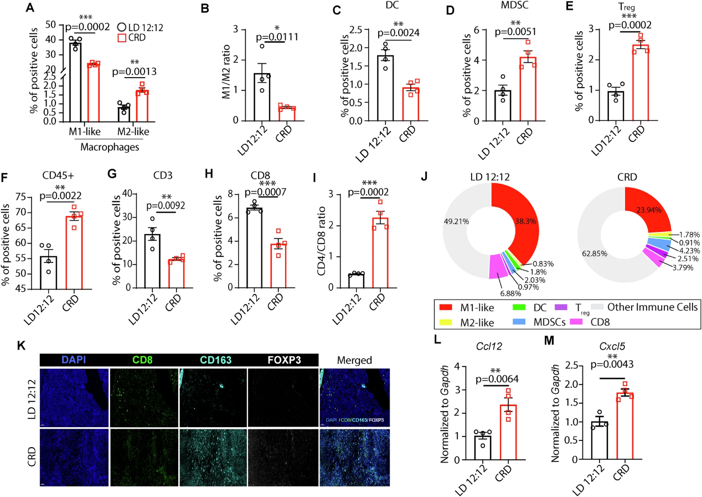
Further research found that the harm of circadian rhythm disruption goes beyond accelerating tumor growth; it also fundamentally damages the structure of breast tissue. Mice with long-term disruption showed reduced branching of mammary ducts, decreased numbers of terminal buds, and also exhibited precancerous lesions such as ductal hyperplasia and disruption of the myoepithelial cell layer, essentially "paving the way" for cancer development. More importantly, circadian rhythm disruption reshapes the tumor microenvironment, transforming the "immune battlefield" that could fight cancer into an "immune desert": it reduces the infiltration of anti-tumor M1 macrophages and CD8+ cytotoxic T cells, while increasing immunosuppressive M2 macrophages, regulatory T cells (Treg), and myeloid-derived suppressor cells (MDSCs), allowing the immune system to "turn a blind eye" and cancer cells to proliferate unchecked.
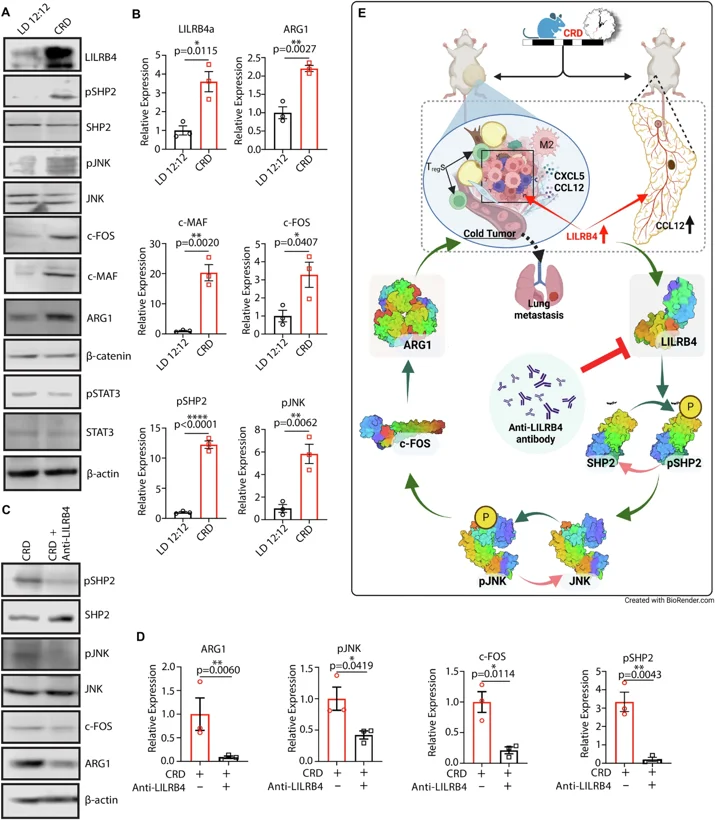
Using single-cell RNA sequencing and other techniques, the research team finally identified the core key molecule: leukocyte immunoglobulin-like receptor B4 (LILRB4). Under normal circumstances, LILRB4 moderately inhibits excessive inflammation and protects healthy tissues, but in tumors with disrupted circadian rhythms, it is abnormally activated, becoming the "off switch" for the immune system.
Experiments showed that in mice with disrupted circadian rhythms, LILRB4 expression levels were significantly elevated in breast tissue and tumors. It activates the JNK-c-FOS-c-MAF signaling axis through the non-canonical WNT signaling pathway, ultimately upregulating immunosuppressive molecules such as ARG1, further reinforcing the "cold tumor" characteristics-these tumors, due to a lack of immune cell infiltration, respond poorly to conventional treatment and have a higher risk of metastasis.
Even more encouragingly, targeted therapy against LILRB4 showed significant effects. After injecting anti-LILRB4 antibodies into mice with disrupted circadian rhythms, researchers found that although tumor volume did not change significantly, the number of lung metastases was greatly reduced, and the immunosuppressive state of the tumor microenvironment was significantly improved: the proportion of Treg cells decreased, ARG1 expression was inhibited, and the immune system regained its ability to fight cancer cells. This finding confirms that LILRB4 is a key link connecting circadian rhythm disruption and breast cancer metastasis, and is a highly promising therapeutic target.
To verify the clinical relevance of the research, the team analyzed the TCGA dataset of human breast cancer patients and found that in the tumor tissue of triple-negative breast cancer patients, the expression of core circadian clock genes (such as PER1, PER2, and CRY1) was significantly downregulated, and the circadian rhythm disruption score of malignant cells was far higher than that of non-malignant cells, confirming that the findings from the mouse model are also applicable to humans. This means that circadian rhythm disruption is not merely associated with cancer risk, but actively drives cancer progression, particularly affecting aggressive, high-mortality triple-negative breast cancer.
"Cancer keeps time, and when the internal clock is disrupted, it takes advantage," Dr. Sarkar emphasized. This study not only reveals the deep connection between late nights, shift work, and breast cancer, but also redefines regular sleep patterns as a crucial factor in cancer prevention and treatment. This finding is an important warning for high-risk groups such as night owls, shift workers, and frequent trans-time zone travelers; and the potential of LILRB4-targeted therapy offers a new treatment approach for these sleep-related cancers.
Creative Bioarray is the leading preclinical CRO specializing in animal models services. We have a wide range of transgenic and inducible mouse and rat models readily available for your studies. Our team of animal model scientists will work with you to find the right model.
Learn more about our fully characterized and validated animal models.
Reference:
- Ogunlusi, Olajumoke, et al. "LILRB4 regulates circadian disruption-induced mammary tumorigenesis via non-canonical WNT signaling pathway." Oncogene (2025): 1-14.

Your email address will not be published. Required fields are marked *