CAR-T Has Emerged as A New Force in Autoimmune Diseases. What Challenges Will It Face?
CAR-T cell therapy has achieved remarkable results in the field of hematological malignancies and is expanding to clinical trials for solid tumors, infectious diseases, and autoimmune diseases.
In recent years, CAR-T cell therapy has successfully treated a variety of autoimmune diseases including systemic lupus erythematosus, idiopathic inflammatory myopathy, myasthenia gravis, and multiple sclerosis. CAR-T cell therapy for autoimmune diseases was also selected as one of the top ten scientific breakthroughs of Science in 2024.
The application of CAR-T cell therapy in autoimmune diseases began with Professor Georg Schett of the University of Erlangen-Nuremberg in Germany. In 2021, the team led by him successfully treated a patient with relapsed/refractory systemic lupus erythematosus (SLE) for the first time using CAR-T cell therapy, opening up a new direction for CAR-T cell therapy.
Recently, Professor Georg Schett published a review paper entitled: Advancements and challenges in CAR T cell therapy in autoimmune diseases in Nature Reviews Rheumatology, a review journal under Nature.
This review comprehensively interprets the progress and challenges of CAR-T cell therapy in autoimmune diseases, including which autoimmune diseases are suitable for CAR-T? What are the applicable targets? What other issues are worth considering in terms of safety, effectiveness and patient management?
As we all know, CAR is inserted into immune cells through genetic engineering, and the extracellular region consists of a single immunoglobulin variable chain for antigen binding, and the intracellular region activates effector cells. At present, in the treatment of autoimmune diseases, most of the patients' autologous T cells are selected, and CAR is introduced through lentiviral vectors.
New CAR constructs are being developed with the aim of selectively eliminating B cells that express specific antigens. After infusion, CAR-T cells rapidly bind to and kill pathological B cells in autoimmune diseases and undergo initial expansion. At the same time, CAR-T cells can also continuously and effectively bind to target cells and eliminate them.
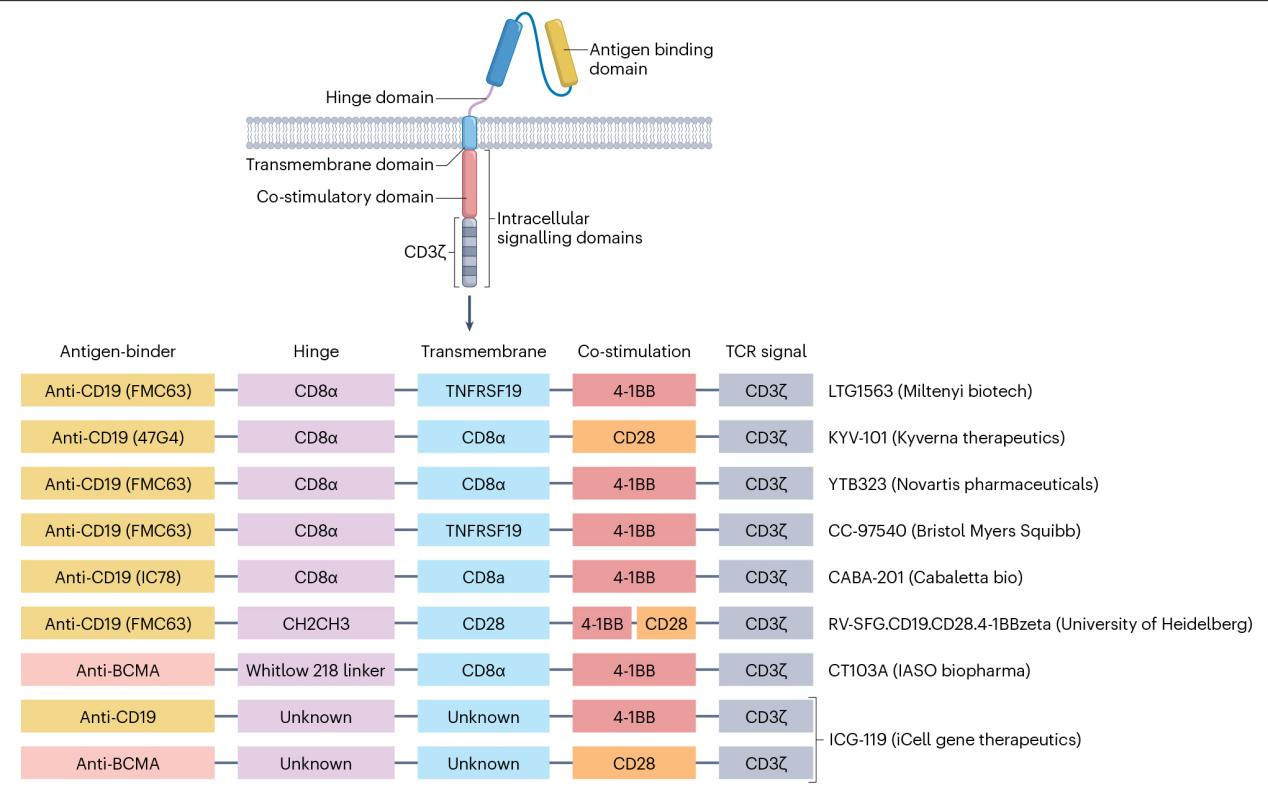 Fig. 1. Vector constructs used for CAR T cell generation in autoimmune disease.
Fig. 1. Vector constructs used for CAR T cell generation in autoimmune disease.
CAR-T can deeply deplete B cells. The depletion of B cells is not only conducive to the elimination of plasmablasts that produce autoantibodies, but also can regulate disease inflammation by abolishing the antigen presentation function of B cells and cytokines. Therefore, CAR-T cell therapy may have unexpected effects in autoimmune diseases with B cells and autoantibodies as the core of the disease, such as systemic lupus erythematosus, idiopathic inflammatory myopathy, systemic sclerosis, etc.
For autoimmune diseases such as psoriasis, inflammatory bowel disease, and spondyloarthritis caused by abnormal T cell activation and IL-23 and IL-17-mediated inflammation, CAR-T cells are difficult to respond and may require more appropriate cell therapies, such as Treg cells or mesenchymal stem cell transplantation.
Evaluating the effect of CAR-T cell therapy depends not only on the physiological mechanism of the disease, but also on whether there are measurable and reliable clinical endpoints. For example, it takes several years for patients with multiple sclerosis to prove that the disease attacks are reduced or disappeared, and long-term research is challenging.
CAR-T target selection is equally important. Most CAR-T cells for the treatment of autoimmune diseases now target CD19 or BCMA. CD19 is highly specific to the B cell lineage and is expressed throughout the B cell differentiation pathway. BCMA is preferentially expressed in mature B cell populations, including memory B cells, plasmablasts, and plasma cells, and may be more advantageous.
CD38 is also a target worth paying attention to for CAR-T. It is expressed on plasma cells, early B cells, T cell subsets, and NK cells, and can provide different target cell spectra. Targeting CD38 does not affect mature B cells, but targets antibody-producing cells, which has advantages in clearing B cells in the early differentiation stage.
In general, CAR-T has "crossed over" from the field of oncology to autoimmune diseases and is rewriting its treatment rules. Developing more targets or dual-target combinations to cover a wider range of B cell lineages can reduce the off-target risk of CAR-T cell therapy and improve safety and effectiveness.
Reference
Schett, Georg, et al. "Advancements and challenges in CAR T cell therapy in autoimmune diseases." Nature Reviews Rheumatology 20.9 (2024): 531-544.

Your email address will not be published. Required fields are marked *