Breaking News: Highly Vascularized Lung and Intestinal Organoids Constructed From iPSCs For The First Time
In 2009, Hans Clevers and others from the Hubrecht Institute in the Netherlands used adult stem cells from the mouse intestine to cultivate the first intestinal organoid, ushering in the era of organoid research. Since then, research results in the field of organoids have continued, and many new and more complex organoids have continued to emerge, bringing more powerful tools to the fields of novel drug development, precision treatment, and regenerative medicine.
However, current organoids generally lack organ-specific and functional vascular networks, especially endodermal organs such as lungs and intestines. The lack of effective vascularization methods has led to the immaturity of the structure and function of lung and intestinal organoids, greatly limiting their application in disease modeling and therapeutic applications.
On June 30, 2025, the Gu Mingxia team of Cincinnati Children's Hospital/UCLA and the Guo Minzhe team of Cincinnati Children's Hospital collaborated to publish a research paper titled: Co-development of Mesoderm and Endoderm Enables Organotypic Vascularization in Lung and Gut Organoids in Cell.
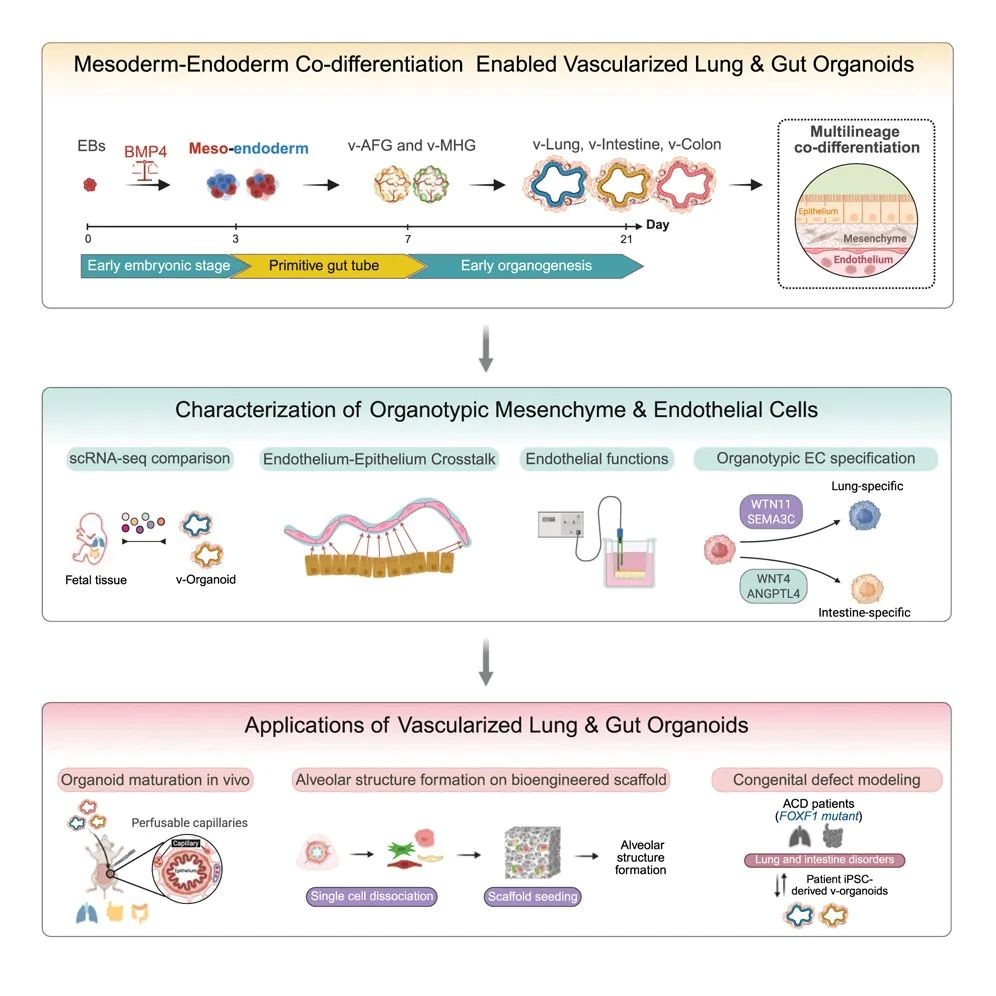 Fig. 1. BMP-mediated mesoderm-endoderm co-differentiation enable vascularized lung & gut organoids.
Fig. 1. BMP-mediated mesoderm-endoderm co-differentiation enable vascularized lung & gut organoids.
This study successfully constructed highly vascularized lung organoids and intestinal organoids for the first time using human induced pluripotent stem cells (iPSCs). These organoid models not only simulate the complex process of early multi-embryonic co-development of human embryos, but also break through the bottleneck of traditional organoids lacking functional blood vessels and organ-specific mesenchyme, providing an advanced platform for studying complex intercellular communication in human organ development and disease and regenerative medicine.
The human vascular system shows significant specialization in different organs to meet local physiological needs. This functional specialization is also evident at the transcriptomic level, as demonstrated by recent human fetal and adult cell atlases, where endothelial cells (ECs) from different organ systems form separate clusters that highlight unique gene signatures.
It has been shown that the unique microenvironment within each organ plays a key role in driving organ-specific vascular endothelial and mesenchymal development. However, the spatial and temporal dynamics of organ vascularization, a complex and highly interactive process, during human development remain poorly understood. Addressing this knowledge gap requires in vitro platforms that incorporate not only relevant cell lineages but also organotypic vasculature with precise cellular configurations.
Organoids are three-dimensional (3D) tissue structures that mimic the cellular composition and function of an organ, creating a microenvironment suitable for studying human development and disease, especially organ-specific vascular development.
Several research groups have focused on organoid assembly methods that involve the fusion of multiple cell types after their respective differentiation. For example, previous studies have attempted to vascularize liver and brain organoids by introducing primary endothelial cells such as human umbilical vein endothelial cells (HUVECs). However, this approach resulted in a lack of clear organ specificity and architecture of endothelial cells, which limited the morphogenesis and functional maturation of organoids. These assembly methods cannot accurately reflect the development of vascular cells during organogenesis in vivo.
In contrast, during early development, coordinated interactions between multiple germ layers guided by morphogenetic patterning signals are essential for the specification and differentiation of organ-specific endothelial and mesenchymal cells. Therefore, reproducing these complex developmental interactions is essential for establishing physiologically relevant vascularization of organoids.
However, vascularization of organoids derived from non-mesodermal lineages, such as ectoderm and endoderm, has been particularly challenging. This is because differentiating human iPSCs into mesodermal lineages versus non-mesodermal lineages requires different signaling pathways. As a result, efforts to vascularize endodermal organoids, such as intestinal and lung organoids, have not yet made substantial breakthroughs.
To address this problem, previous studies have attempted to generate vascularized intestinal organoids by expanding the small number of endogenous endothelial progenitor cells that occasionally appear during endoderm differentiation by introducing pro-angiogenic factors such as bone morphogenetic protein-4 (BMP4), vascular endothelial growth factor A (VEGFA), fibroblast growth factor (FGF), or epiregulin (EREG). Although the endothelial cells cultured by these methods partially reflect the genetic characteristics of human fetal small intestinal endothelial cells, the resulting blood vessels are still limited in number, immature in structure and function, and often lack organ-specific characteristics and complete function. More importantly, existing lung organoid generation methods have not successfully recapitulated the human alveolar-capillary interface, which is critical for gas exchange. These limitations are also expected, as most protocols for differentiating foregut and lung organoids from iPSCs rely on strong inhibition of bone morphogenetic protein (BMP) and transforming growth factor-β (TGF-β) signaling pathways. While these conditions are effective for endoderm patterning, they are incompatible with the specialization of vascular and mesenchymal lineages. Therefore, the lack of robust vascularization in intestinal and lung organoids greatly limits their physiological relevance for disease modeling and therapeutic applications.
In this latest study, the research team establish an in vitro vascularized organoid platform that truly reproduces the coordinated development of mesoderm and endoderm lineages. This method can achieve efficient differentiation and lineage specialization of endoderm derivatives and organotypic endothelial/mesenchymal cell populations.
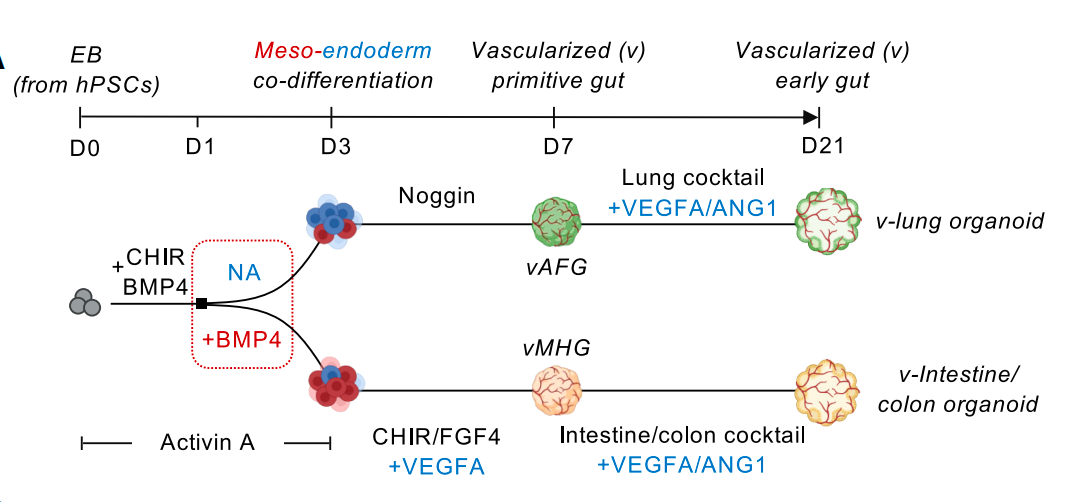 Fig. 2. Co-differentiated mesoderm and endoderm within the same spheroid to vascularize lung and intestinal organoids from induced pluripotent stem cells.
Fig. 2. Co-differentiated mesoderm and endoderm within the same spheroid to vascularize lung and intestinal organoids from induced pluripotent stem cells.
Traditional methods usually require the addition of more than a dozen factors, while in this method, only one inhibitor, Noggin, is required for the culture of lung organoids, and only three activators, CHIR99021, FGF4, and VEGFA, are required for the culture of intestinal organoids. Cells in 3D culture spheres spontaneously secrete signaling molecules necessary for vascular growth, thereby naturally forming a vascular network. The resulting vascularized lung and intestinal organoids have organ-specific endothelial and mesenchymal elements, showing enhanced cell type diversity, three-dimensional structure, improved cell survival and maturity, and exhibiting real physiological functions - pulmonary blood vessels form a tight barrier (simulating gas exchange), and intestinal blood vessels show high permeability (facilitating nutrient absorption). After transplantation into mice, the organoid blood vessels integrate with the host circulatory system while maintaining organ specificity, further promoting organoid maturation and successfully achieving blood perfusion, marking the first time that organoid blood vessels have in vivo circulation capabilities.
This multi-lineage organoid system can be used to study abnormal cell-cell interactions in different disease contexts. Alveolar capillary dysplasia with malposition of pulmonary veins (ACDMPV) is a congenital lung disease caused by mutations in the mesoderm FOXF1 gene. However, traditional lung epithelial organoids lack mesenchymal and endothelial cells that express FOXF1, which limits the ability of these models to simulate this lung disease. By differentiating iPSCs from patients with FOXF1 gene mutations into vascularized lung organoids, the research team was able to reproduce the primary endothelial defects and secondary epithelial abnormalities caused by interrupted endothelial-epithelial interactions in the disease. The synchronously constructed intestinal organoids also reproduced the intestinal malrotation associated with patients, and for the first time, the same platform was used to simulate multi-organ interaction diseases, which brought hope to complex diseases that traditional models could not crack.
Highlights of This Study:
BMP-mediated co-differentiation of mesoderm-endoderm is essential for organ-specific vascular systems;
Organ-specific vascular systems support the development and maturation of endoderm organoids;
Vascularized organoids have the characteristics of human fetal lungs and intestines;
The vascularized organoid simulates the abnormal endothelial-epithelial cell interactions in FOXF1 disease.
Reference
Miao, Yifei, et al. "Co-development of mesoderm and endoderm enables organotypic vascularization in lung and gut organoids." Cell (2025).

Your email address will not be published. Required fields are marked *