Sonogenetic EchoBack-CAR T Cells: A Universal, Efficient and Safe Strategy for Solid Tumor Treatment
CAR-T cell therapy has shown "curative" potential in blood tumors such as leukemia. However, in solid tumors, which account for the vast majority of cancers, CAR-T cell therapy has repeatedly hit a wall — off-target toxicity, T cell exhaustion, short-term efficacy and other issues have become insurmountable walls.
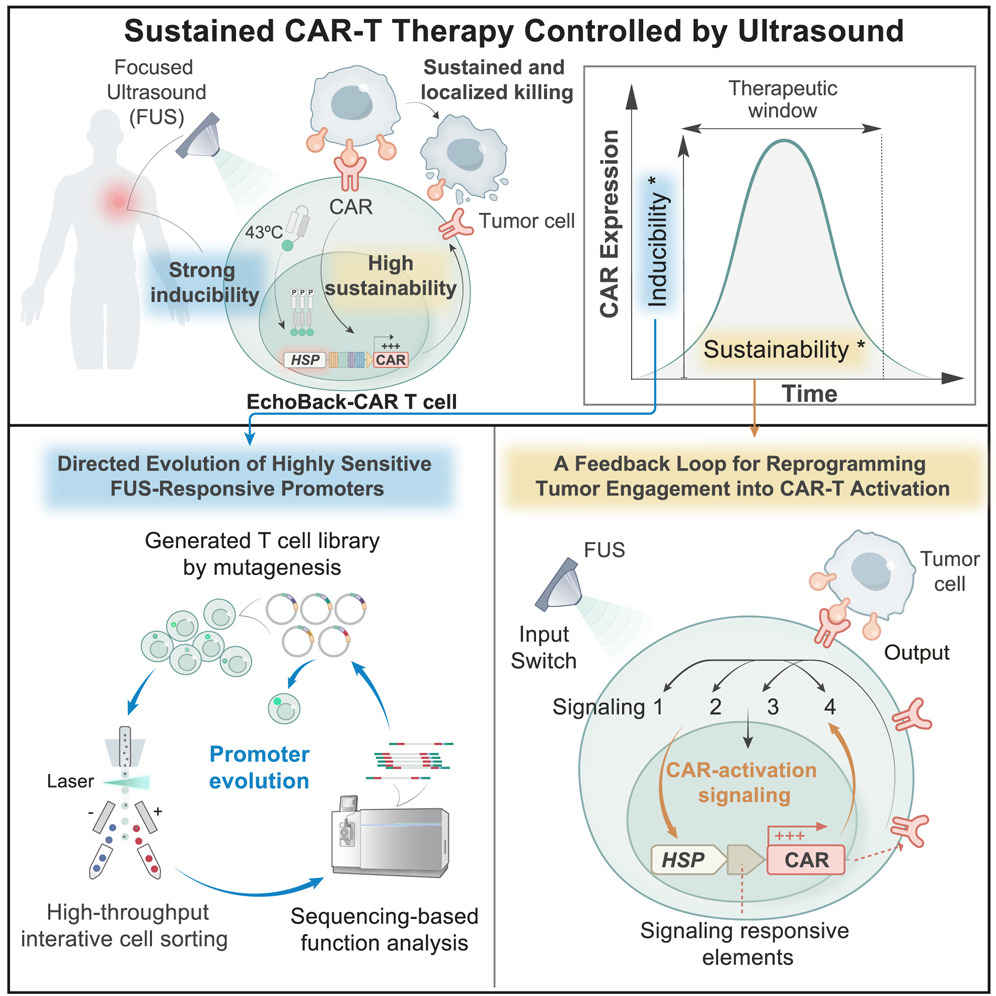
Recently, a breakthrough study published in the journal Cell has brought a new answer to CAR-T cell therapy for solid tumors — EchoBack-CAR-T cells, which can be precisely regulated by ultrasound to achieve accurate and lasting therapeutic effect.
Three major technological breakthroughs of EchoBack-CAR-T are as follows:
1. Ultrasonic remote-control switch: Find the most sensitive "gene switch"
The research team successfully screened out an ultra-sensitive heat shock promoter from millions of candidate promoters through high-throughput screening technology. This "switch" can be activated under local heating (ultrasonic precision control) at 43°C (compared with traditional promoters, the induction efficiency is increased by 5 times and the response speed is shortened to 15 minutes), driving the persistent expression of CAR protein, and there is almost no leakage at normal body temperature, greatly reducing the risk of off-target.
2. Self-reinforcing "echo feedback" loop
Once CAR-T cells recognize tumors, their activation signals (such as NFAT and NF-κB pathways) will be converted into instructions for continuous production of CAR, forming a positive feedback loop. This design cleverly solves the problem of "activation is exhaustion".
In vitro experiments: CAR expression can last for more than 3 days, while traditional CAR disappears within 24 hours.
3D glioblastoma model: After two ultrasound stimulations, the tumor volume was reduced by 90% (while traditional CAR only suppressed it temporarily).
3. Modular design: a universal platform that "kills two birds with one stone"
The research team applied the same technology to two solid tumor targets - glioblastoma (targeting GD2) and prostate cancer (targeting PSMA), both of which showed significant efficacy, proving that the platform can be quickly adapted to different cancer types.
In the glioblastoma mouse model, the survival of mice in the EchoBack-hGD2CAR-T cell treatment group was as high as 100%, and no off-target toxicity occurred, while all mice in the control group died within 20 days. Single-cell sequencing results showed that compared with traditional CAR-T cells, EchoBack-CAR-T cells had enhanced cytotoxicity and reduced exhaustion.
In the prostate cancer mouse model, EchoBack-PSMACAR-T cells achieved long-term tumor suppression (tumor volume was reduced by 80%), with minimal off-target toxicity, and distal low-PSMA-expressing tissues were completely unattacked, achieving precise attack on tumor tissues.
These results suggest that Sonogenetic EchoBack-CAR T cells can be used as a universal, efficient, and safe strategy for solid tumor treatment.
Why is it safer and more durable?
Spatiotemporal control: Focused-ultrasound (FUS) is used to induce temperature rise only locally in the tumor, so CAR expression is limited to the target area.
Periodic rest: The interval between two stimulations allows T cells to rejuvenate and avoid chronic exhaustion.
Clinical compatibility: No leakage at 41°C (simulated fever), and short-term heating at 43°C does not damage normal tissues.
Reference
- Liu, Longwei, et al. "Engineering sonogenetic EchoBack-CAR T cells." Cell (2025).

Your email address will not be published. Required fields are marked *