Overview of NDA in Q3 2017 by FDA: ‘Blockbuster’ Potential for Nearly Half of 11 New Drugs
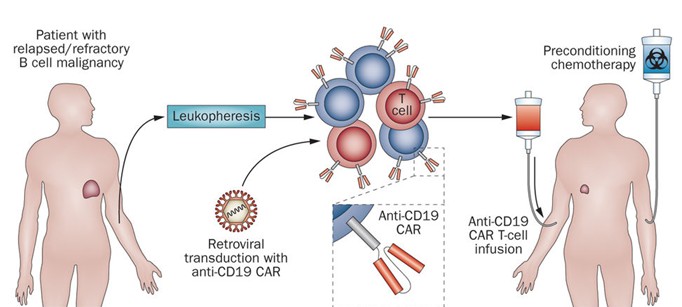
Abstract Time has arrived in the fourth quarter of 2017. There is no doubt that time is the most objective judge and the great things will always withstand the tempered time. FDA approved the adoption of a variety of highly anticipated drugs in the third quarter of 2017. In general, FDA approved 11 new drugs, which includes a CAR-T cell product KYMRIAH approved by the Center for Biologics Evaluation and Research (CBER). The following is an overview of the new drug information approved in Q3 2017, also involving introduction for several drugs that attract much attention therein. 2017 Q3: New Drugs with optimistic market prospects There are 11 new drugs being approved in 2017 Q3, and the number of approved contracts was flat with the previous two quarters. As of September 27, 2017, a total of 34 new drugs were approved, of which a variety of drugs are regarded as ‘ born with a silver spoon in their mouth’. Such as J & J’s new psoriasis drug IL-23 monoclonal antibody (Mab): guselkumab, world’ s first CAR T-cell product KYMRIAH produced by Novartis, and HCV cocktail therapy of Gilead/AbbVie. We will detail the specifics of each type of drug in this post: 1.J & J’s new psoriasis drug IL-23 monoclonal antibody (Mab): guselkumab At present, Humira, Ustekinumab, Etanercept, Daivobet (calcipotriol/betamethasone) and Infliximab dominate the psoriasis drug market, and guselkumab will continue to enhance Johnson & Johnson's voice in the psoriasis market, as it brings more clinical benefits that those from Humira. Market research institutions EvaluatePharma predicted drug sales of 2022 is expected to reach 1.6 billion dollars. 2.First extended adjuvant therapy for Breast cancer: Neratinib maleate will reduce the risk of death 33% The drug is the first approved adjuvant therapy for this type of breast cancer, adjuvant therapy for HER2-positive breast cancer patients is an important part of the treatment plan, the drug also received a very positive market expectations that 2022 sales are expected to break through 1.25 billion dollars. 3.Cocktail therapy of Gilead/AbbVie Currently, the U.S. HCV drug market is shrinking rapidly, and effective patients are sharply reduced due to the great complete remission rate of direct antiviral drugs. That’s why we need to analyze how to survive in this crisis. One is to develop new HCV products for the treatment of patients that get refractory hepatitis C, the other is to expand overseas HCV market actively. Such as China, the prelude has been kicked off that Gilead and AbbVie competing to land the Chinese market. HCV Cocktail Therapy offered by Gilead and AbbVie has provided a new treatment option for patients with chronic hepatitis C, sofosbuvir or NS5A inhibitors. Evaluatepharma also place great expectations on this two products. It is expected that that sales of Gilead’ s Vosevi will break through 1.14 billion US dollars while AbbVie’ s Mavyret will reach 1.25 billion dollars by 2022. 4.A new era: Novartis’ KYMRIAH goes down in history The Center for Biologics Evaluation and Research (CBER) approved the world's first CAR-T cell product KYMRIAH in Aug 30, 2017. Novartis’ product and Kite Pharma’ s product of the same kind has always been the focus on attention. Although the current cell products are still imperfect due to pricing controversial, KYMRIAH has brought us into a new era and provided the miracle of life for some leukemia patients, and the drug sales is expected to USD 1.0 billion in 2022. All the mentioned above is about some information about the drug approved in 2017 Q 3. The remarkable effect for the drugs is commendable and the quality of those drugs is quite high.

Your email address will not be published. Required fields are marked *